Case Control Studies
Table Of Content
In particular, issues arising from timing, research biases like recall bias, and the selection of variables lead to low internal validity and the inability to determine causality. Case-control studies are a type of observational study often used in fields like medical research, environmental health, or epidemiology. While most observational studies are qualitative in nature, case-control studies can also be quantitative, and they often are in healthcare settings. Case-control studies can be used for both exploratory and explanatory research, and they are a good choice for studying research topics like disease exposure and health outcomes. Case-control studies in India tend to be poor in quality because they are based on smallsample sizes. Small samples do not have sufficient statistical power to adjust for themultitude of confounding variables that bedevil research in psychiatry.
Fast and optimal algorithm for case-control matching using registry data: application on the antibiotics use of colorectal ... - BMC Medical Research Methodology
Fast and optimal algorithm for case-control matching using registry data: application on the antibiotics use of colorectal ....
Posted: Fri, 02 Apr 2021 07:00:00 GMT [source]
Analysis of case-control studies
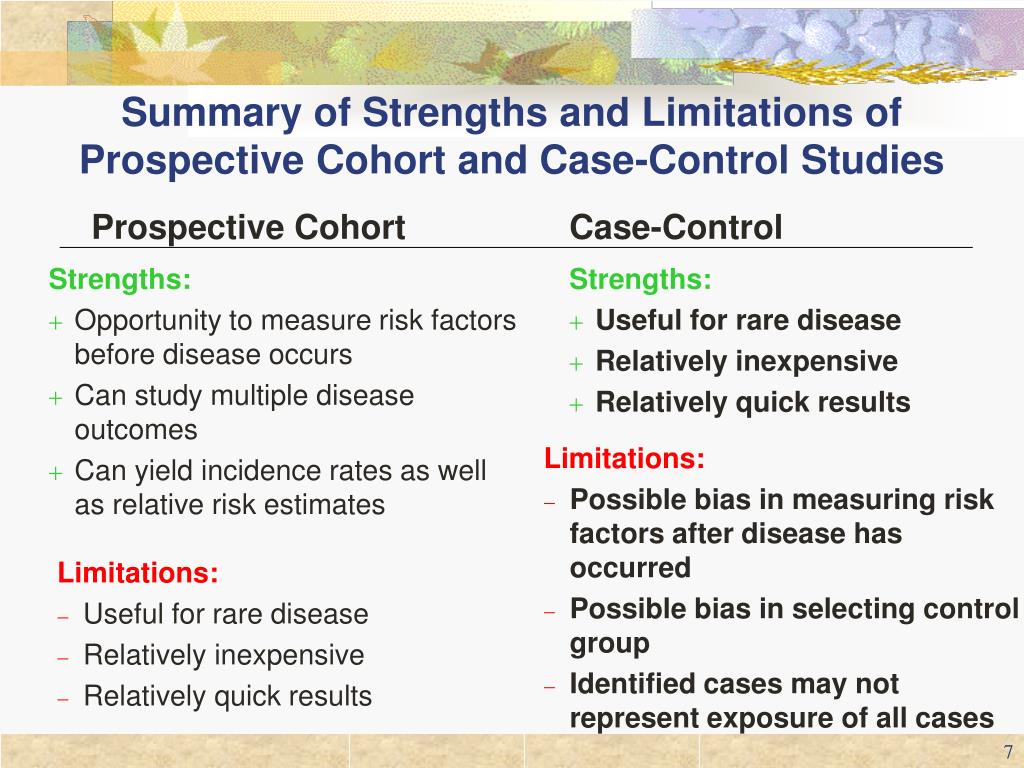
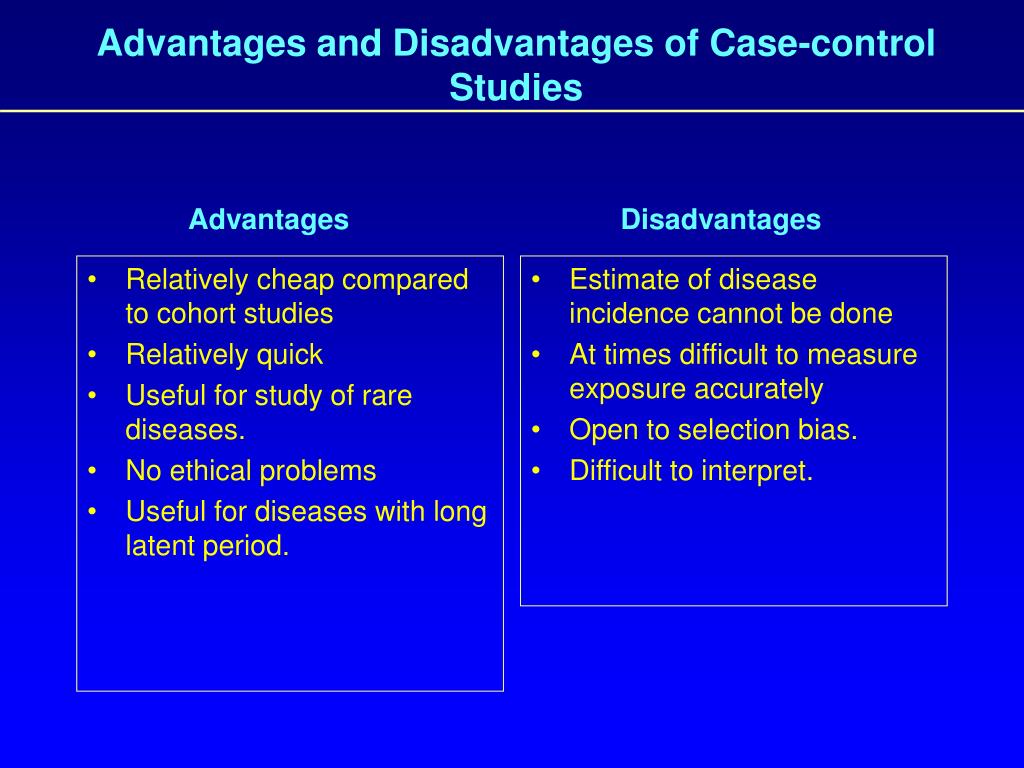
This measure is the ratio of the odds of an exposure between cases and controls and in most cases approximates the relative risk. As in a cohort study, the analytic plan for a case–control study typically involves advanced statistical methods to adjust for multiple potential confounders. A case-control study is a good tool for exploring risk factors for rare diseases or when other study types are not feasible. Many times an investigator will hypothesize a list of possible risk factors for a disease process and will then use a case-control study to see if there are any possible associations between the risk factors and the disease process.
Analysis of Case-Control Studies
For example, in the above mentioned Melanoma and Tanning study, the researchers defined their population as any histologic variety of invasive cutaneous melanoma. However, they added another important criterion – these individuals should have a driver's license or State identity card. This probably is not directly related to the clinic condition, so why did they add this criterion?
Similar articles
The collected data were reviewed and checked for omissions, completeness, and consistency by the data collectors and principal investigator on daily bases during data collection time. All perinatal deaths that occurred at ACSH were the source population of the study. All perinatal deaths that occurred between January 01 to December 31, 2020 at ACSH were the study population.
Clinical Significance
For example, Hux and others19 validated definitions of diabetes by comparing International Classification of Diseases codes obtained from administrative health care databases in Ontario with diagnostic data from primary care charts. When designing a case-control study, the researcher must find an appropriate control group. Ideally, the case group (those with the outcome) and the control group (those without the outcome) will have almost the same characteristics, such as age, gender, overall health status, and other factors.
A confidence interval that includes 1.0 means that the association between the exposure and outcome could have been found by chance alone and that the association is not statistically significant. Case-control studies cannot provide any information about the incidence or prevalence of a disease because no measurements are made in a population based sample. Historically controlled studies can be considered as a subtype of non‐randomized clinical trial. In this study design subtype, the source of controls is usually adopted from the past, such as from medical records and published literature.1 The advantages of this study design include being cost‐effective, time saving and easily accessible. However, since this design depends on already collected data from different sources, the information obtained may not be accurate, reliable, lack uniformity and/or completeness as well. Though historically controlled studies maybe easier to conduct, the disadvantages will need to be taken into account while designing a study.
Measuring Occurrence of Outcomes
In a case-control study, it is imperative that the investigator has explicitly defined inclusion and exclusion criteria prior to the selection of cases. For example, if the outcome is having a disease, specific diagnostic criteria, disease subtype, stage of disease, or degree of severity should be defined. Second, cases may be selected from a variety of sources, including hospital patients, clinic patients, or community subjects. Many communities maintain registries of patients with certain diseases and can serve as a valuable source of cases. However, despite the methodologic convenience of this method, validity issues may arise.
Related Articles
For a study to be classified as a case-control study, the study should be an observational study and the participants should be recruited based on their outcome status (some have the disease and some do not). If, for example, our cases of Kaposi's sarcoma came from across the country but our controls were only chosen from a small community in northern latitudes where people rarely go outside or get sunburns, asking about sunburn may not be a valid exposure to investigate. Similarly, if all of the cases of Kaposi's sarcoma were found to come from a small community outside a battery factory with high levels of lead in the environment, then controls from across the country with minimal lead exposure would not provide an appropriate control group. The investigator must put a great deal of effort into creating a proper control group to bolster the strength of the case-control study as well as enhance their ability to find true and valid potential correlations between exposures and disease states. A non‐randomized clinical trial involves an approach to selecting controls without randomization.
Care must be taken with sampling to ensure that the controls represent a ‘normal’ risk profile. Another use for case-control studies is investigating risk factors for a rare disease, such as uveal melanoma. Patients who present to hospital, however, may not be representative of the population who get melanoma.
These types of studies, along with randomised controlled trials, constitute analytical studies, whereas case reports and case series define descriptive studies (1). Although these studies are not ranked as highly as randomised controlled trials, they can provide strong evidence if designed appropriately. The advantage with this methodology is that it enables comparability between experiment/intervention groups and thus makes result analysis more efficient.
The case group would consist of all those patients at the hospital who developed post-operative endophthalmitis during a pre-defined period. The procedures used for the collection of exposure data should be the same for cases and controls. Case-control studies are a solid research method choice, but they come with distinct advantages and disadvantages. You would then collect information on any history of early life stress (e.g., abuse, neglect, trauma) for both the cases and controls and compare the two groups to determine if there is a relationship between early life stress and the risk of developing PTSD.
Formulation of a clearly defined hypothesisAs with all epidemiological investigations the beginning of a case-control study should begin with the formulation of a clearly defined hypothesis. Case definition It is essential that the case definition is clearly defined at the outset of the investigation to ensure that all cases included in the study are based on the same diagnostic criteria. A case-control study is an experimental design that compares a group of participants possessing a condition of interest to a very similar group lacking that condition. Here, the participants possessing the attribute of study, such as a disease, are called the ‘case’, and those without it are the ‘control’. Case Control Studies are prospective in that they follow the cases and controls over time and observe what occurs. This study would be retrospective in that the former lifeguards would be asked to recall which type of sunscreen they used on their face and approximately how often.
Furthermore, only new (incident) cases should be selected, as nonincident cases usually over-represent long-term survivors, and diagnostic practices may change over time, introducing potential bias. When cases are selected from a secondary data source, the case definitions should be supported by previous validation studies. The weaknesses of case–control studies include inefficiency for studying rare exposures, difficulty of selecting unbiased controls, and inability to directly calculate incidence rates of outcomes. The most commonly cited disadvantage in case-control studies is the potential for recall bias. Recall bias in a case-control study is the increased likelihood that those with the outcome will recall and report exposures compared to those without the outcome. In other words, even if both groups had exactly the same exposures, the participants in the cases group may report the exposure more often than the controls do.
A main advantage is that these controls are likely to satisfy the ‘study-base’ principle (described above) as suggested by Wacholder and colleagues. Furthermore, many of these controls will not be inclined to participate in the study; thus, the response rate may be very low. According to them, an important aspect of selecting a control is that they should be from the same ‘study base’ as that of the cases. Thus, the pool of population from which the cases and controls will be enrolled should be same. For instance, in the Tanning and Melanoma study, the researchers recruited cases from Minnesota Cancer Surveillance System; however, it was also required that these cases should either have a State identity card or Driver's license.
Comments
Post a Comment